Science Snapshot – Gene Therapy
Author: Tierney Baum, PhD, CSNK2A1 Foundation Strategic R&D Consultant
Edited by: Gabrielle Rushing, PhD, CSNK2A1 Foundation Chief Scientific Officer & Alison Kujawski, MPH, Director of Outreach, American Society of Gene + Cell Therapy (ASGCT)
For many disorders the common treatments like medications or surgeries focus on managing symptoms or improving the effects of the condition on a person’s life. Gene therapy, however, is an approach that aims to tackle the root cause of genetic disorders by targeting a gene variant with the goal of slowing or stopping disease progression. The field of gene therapies has seen a growing number of FDA approved treatments in recent years, with hundreds of clinical trials underway. The research is moving quickly, offering hope for treating many disorders that were once considered untreatable.
What Should Parents Know?
Currently, there are no approved or trial-stage gene therapies specifically developed for patients with OCNDS. However, since OCNDS is caused by just one gene, it’s possible that gene therapies could be developed in the future to potentially improve symptoms or slow or stop disease progression. Participating in natural history studies and other OCNDS research initiatives is vital to help researchers better understand OCNDS so that future treatments such as gene therapy can be developed in the future. Learn more about
participating in OCNDS research here.
ASGCT Glossary
To learn more about the different types of gene therapies you may hear about, the American Society of Gene & Cell Therapy (ASGCT) offers a Gene Therapy Glossary. This resource explains key terms in plain language and can help you better understand how each approach works, where it fits in the broader field of genetic medicine, and how it may relate to current or future treatments. You can explore the glossary here:
ASGCT Gene Therapy Glossary.
What is gene therapy?
Gene therapy works by changing the genes inside a patient’s cells either by replacing, inactivating (silencing), or repairing faulty genes. Currently, we do not have sufficient information to know whether all variants in the CSNK2A1 gene that cause OCNDS would benefit from gene therapy. It is likely that each of these approaches could work depending on the type of variant and which part of the gene is affected.
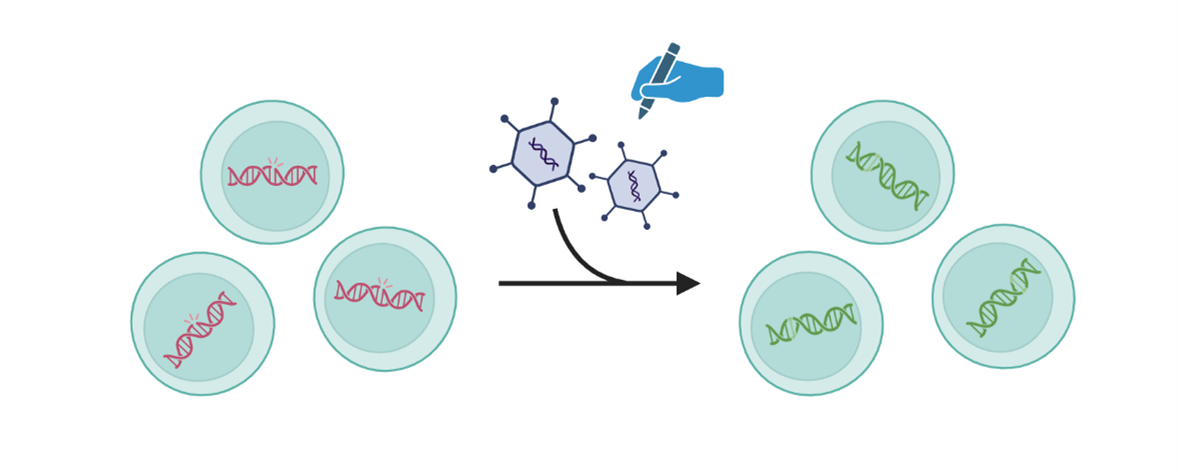
1. Replacing a gene
Gene replacement therapy involves introducing a working copy of a gene to the person’s cells to restore proper cell function. This approach can be used to treat genetic disorders where the variant causes a specific protein to not be produced or function as it should.
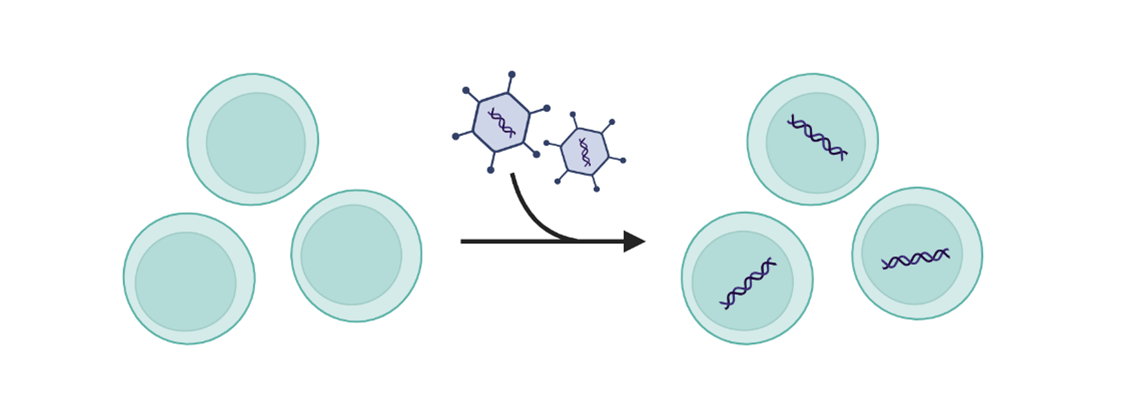
2. Inactivating a gene (gene silencing)
Inactivating gene therapies, or gene silencing, is where the delivered genetic material prevents or inhibits the activity of a gene that is already present in a cell. Gene silencing often decreases the amount of a specific protein being made. For example, most cardiovascular diseases are caused by high LDL-cholesterol and lipoproteins; by inactivating genes that have an important role in the production of lipoproteins it is possible to significantly reduce their levels.
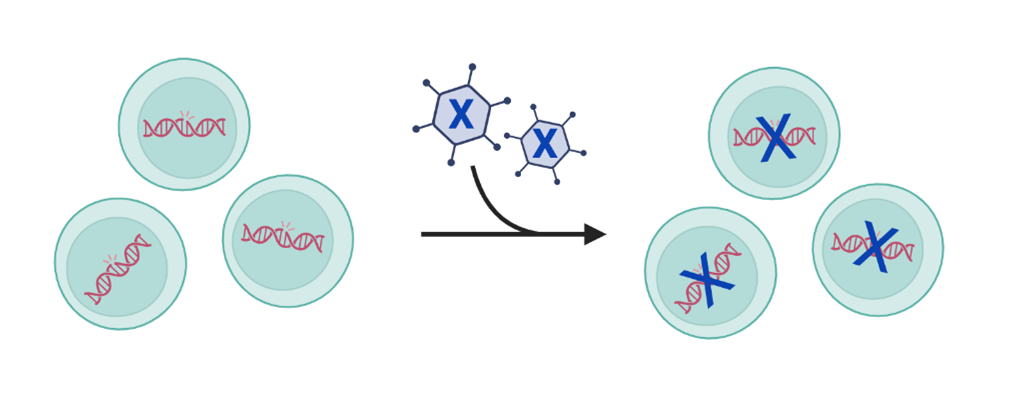
3. Repairing a gene
Repairing a gene, or gene editing, corrects pieces of DNA by changing or deleting the information
within the affected gene. Genetic material is sent to directly edit or change pieces of DNA already located within a cell to correct the protein being made by that DNA. Gene editing uses technology that is highly precise to make these types of changes. After cutting the DNA, the cell naturally repairs itself leading to permanent genetic changes that allow the cell to function normally. Learn more about Gene Editing.
Genes cannot be easily inserted into cells and require a mode of delivery using a carrier called a vector. Vectors are usually viruses because they can recognize specific cells and transfer genetic material into cells. Viruses can be modified to carry disease therapeutic genes rather than viral genes that would normally cause disease. This new genetic material, such as a working gene, is delivered into the cell using a vector. A vector is like a package used to deliver a specific message. Viruses can be used as vectors because they have evolved to be very good at getting into cells. Scientists have learned how to remove the viral genes and use this same ability to treat or prevent disease. In this case, their goal is to insert the new genetic material into the cell. All viral vectors are tested many times for safety prior to being used in humans.
The vector can be delivered in one of two ways:
- ex-vivo treatment removes the person’s own cells and delivers the genetic material to these cells outside the body. The modified cells are then returned to the body.
- in-vivo treatment means the genetic material is delivered directly into the person, such as through an injection.
Why Gene Therapy Matters for Rare Disorders
Many rare genetic disorders are caused by a mutation in a single gene. By fixing the gene, gene therapy could be potentially curative for some disorders, which often have no other treatment options. Rare diseases don’t usually attract the same attention from pharmaceutical companies compared to more common conditions, because they affect fewer people. To encourage research and development, some government programs offer incentives to make it easier to develop treatments for these diseases.
Here are two examples of gene therapies used in rare disorders:
Spinal Muscular Atrophy [gene replacement]
Spinal Muscular Atrophy (SMA) is a genetic disorder that affects the spinal cord and nerves in children, resulting in muscle wasting and weakness that is often fatal. SMA is caused by a disruption in the function of the SMN1 gene. An FDA-approved gene therapy delivers a new, working copy of the SMN1 gene administered one time to children under 2 years old. This gene infusion makes up for the missing or nonfunctional SMN gene and helps motor neurons begin to work properly. This breakthrough therapy has been shown to be very effective in treating SMA.
Duchenne Muscular Dystrophy [gene replacement using a shorted, functional version of the gene]
Duchenne Muscular Dystrophy (DMD) is a disorder that affects children leading to fatigue, weak muscles, and difficulty with movement. DMD typically becomes more severe as children get older and most do not survive past age 30. While there is no cure for DMD, gene therapies have greatly improved symptoms. The injection of AAV vectors is used to deliver dystrophin-producing genes directly into cells in the muscle. The dystrophin helps protect and strengthen muscle fibers, thus improving DMD symptoms.
Challenges & Risks of Developing New Gene Therapies
Even though some gene therapies have been approved by the FDA, many others are still in clinical trials. Developing new therapies is challenging because:
- Getting genetic material into cells: It’s difficult to deliver genes into the right cells in the body.
- Targeting the correct cells or gene: Ensuring that the gene reaches the right location and works properly can be tricky especially when the brain is a desired target due to the blood-brain-barrier.
- Minimizing side effects: Any treatment can come with risks, and gene therapy is no different.
Blood-brain-barrier – a layer of cells that acts as a filter to the brain keeping harmful substances and pathogens out while allowing essential nutrients and oxygen to pass through. It also limits the entry of immune cells to the brain which is why we refer to the central nervous system as ‘immune privileged’.
- Immune system reactions: The body’s immune system might react to the new genes, or the viral vector used to deliver them. The American Society of Gene + Cell Therapy (ASGCT) has a short Q&A on this topic.
- Targeting the wrong cells: If the therapy targets the wrong cells, it could cause unwanted effects.
- New genetic errors: In rare cases, gene editing could introduce new mistakes in the DNA.
- Long-term effects are unknown: This is why gene therapies are monitored in a person for years following treatment.
- No guarantees: There is always a chance that the investigational treatment may not work or may have unexpected side effects.
Because gene therapy is still new, we don’t yet know all the potential risks or how long a treatment may last. However, depending on the severity of the disorder, the benefits might outweigh the risks. Before gene therapy clinical trials begin, they must be reviewed and approved by an Institutional Review Board (IRB), a group of experts who make sure that safety is a priority. The FDA’s Center for Biologics Evaluation and Research (CBER) also regulates gene therapy products to ensure their safety and effectiveness. ASGCT has an overview of the clinical trials process you can review here.
For more information about gene therapy and answers to common questions, check out this video from the National Organization for Rare Disorders (NORD):
Gene Therapy: Your Questions Answered and a talk from our 2024 OCNDS Virtual Symposium by our Scientific Advisory Board member,
Dr. Rachel Bailey. The American Society of Gene + Cell Therapy also has a
Gene Therapy 101 resource.
References
FDA https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/what-gene-therapy
Mayo Clinic https://www.mayoclinic.org/tests-procedures/gene-therapy/about/pac-20384619
National Human Genome Research Institute https://www.genome.gov/genetics-glossary/Gene-Therapy
ASGCT Glossary:
https://patienteducation.asgct.org/gene-therapy-101/glossary?utm_source=chatgpt.com