Science Snapshot – How Drug Repurposing Can Help Rare Disease Communities
Author: Monica Strain, CSNK2A1 Foundation Intern
Edited by: Gabrielle Rushing, PhD, Chief Scientific Officer, CSNK2A1 Foundation
Reviewed by: Elisabeth Mellinger, Parent Advisory Board Member, CSNK2A1 Foundation
In this Science Snapshot, we look at
drug repurposing – a promising strategy that may help bring treatments to rare disease communities faster, including those affected by OCNDS. We’ll explore what drug repurposing is, how it works, and why it holds unique potential for rare disease research and patient outcomes.
What is drug repurposing?
Drug repurposing – also known as drug repositioning - is a strategy for using an existing drug for a new treatment or medical condition for which it was not indicated before. In other words, it’s giving a known drug a new therapeutic purpose. This approach offers several benefits:
- It’s often safer. Since the drug has already been found safe in preclinical models and humans, scientists already understand its safety profile, which lowers the risk of unexpected side effects or failures.
- It saves time. Many of the early stages of drug development - such as testing in animals and safety studies in humans - have already been completed, allowing scientists to progress faster into studies focused on the new indication.
- It can be more cost effective. Developing a brand-new drug can take over a decade and cost billions of dollars. Repurposing an existing drug can significantly reduce both time and expenses – although the savings depends on the specific case.
- It may lead to new scientific discoveries. Repurposing a drug may reveal new targets or biological pathways that can be further explored by scientists, which can accelerate progress towards future therapies.
To summarize, drug repurposing can offer a safer, faster, and more cost-effective option to deliver treatments, which is important for rare diseases, where approved treatments are limited and urgently needed.
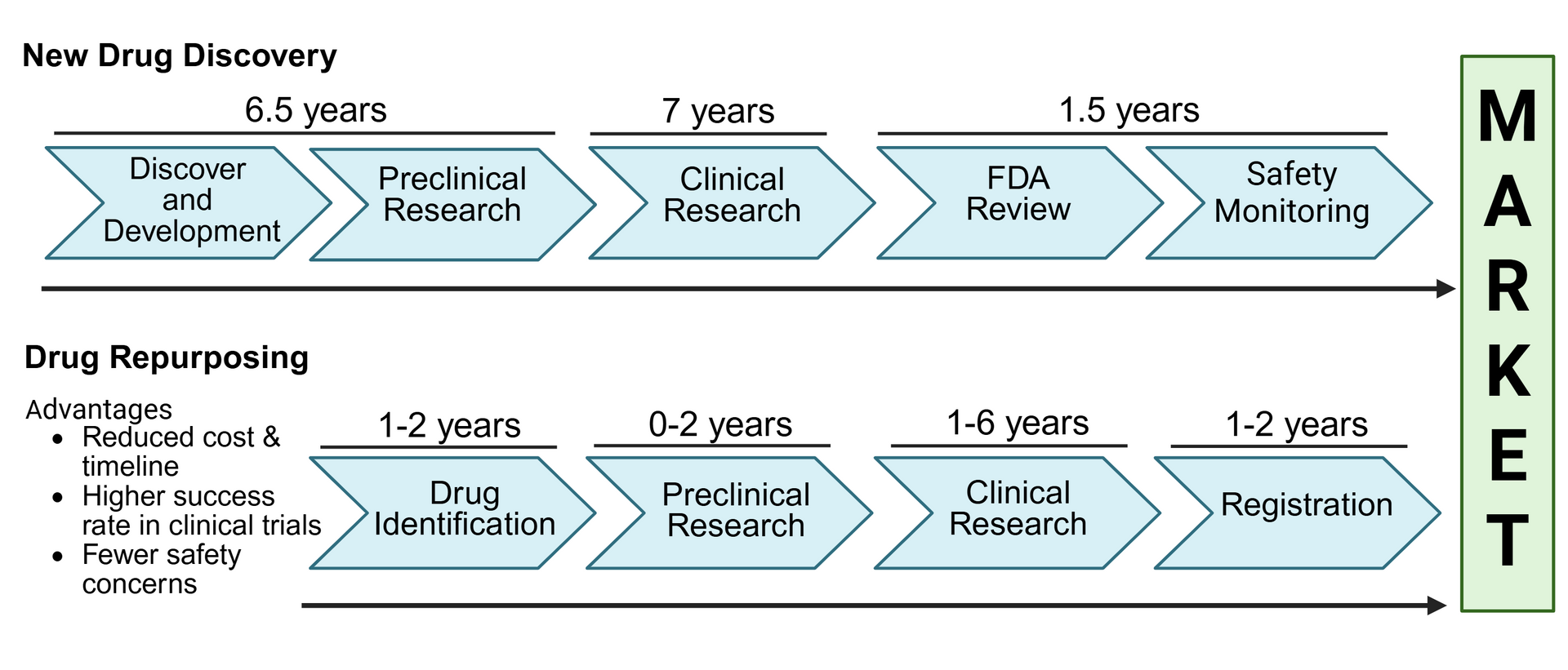
How do scientists identify drug repurposing opportunities?
Scientists use a variety of methods to identify existing drugs that might be effective for new conditions. Some common approaches include:
- Computational screening: Scientists use computer based methods to rapidly analyze large datasets and uncover connections between approved drugs and disease-related genes, proteins, or pathways using specialized bioinformatic tools such as software designed to manage, analyze, and interpret biological data
- High throughput screening: In laboratory settings, scientists systemically test a wide variety of existing drugs on disease models (such as cultured cells and animals) to see if any produce beneficial therapeutic effects. This method allows for rapid evaluation of many candidates at once.
- Clinical observations: Sometimes patterns observed in clinical data (e.g., data from medical records) can provide valuable information about new therapeutic uses for that drug. For example, if patients taking a drug for one condition show unexpected improvements in unrelated symptoms, researchers may explore whether the drug can be repurposed for that new indication.
- Mechanism-based approaches: If an existing drug is known to target a specific biological process that is also involved in a different disease, scientists may investigate whether the drug could be effective in that context. This is useful when the underlying biology of two conditions overlaps.
Once a promising drug candidate is identified, it still needs to undergo clinical studies to assess its effectiveness and safety in the new indication.
Why does drug repurposing matter in rare diseases?
There are over 7,000 known rare diseases, yet fewer than 5% have an approved therapeutic agent by the U.S. Food and Drug Administration (FDA). For many families, this means often waiting years or even decades for therapeutic options.
Drug repurposing offers real promise for rare diseases for several reasons:
- Scientific benefits: Many rare diseases are complex and often poorly understood, with limited research into their underlying mechanisms. Repurposed drugs can help scientists uncover new insights into disease mechanisms by observing how known compounds interact with lesser-known conditions.
- Regulatory support: Agencies like the FDA offer special incentives to encourage rare disease drug development, including Orphan Drug Designation. These incentives such as tax credits, reduced fees, and market exclusivity–make drug repurposing more appealing and feasible for researchers and companies. Market exclusivity means that there is a set period of time when no other company can sell the same drug for the same condition, even if their version is approved. For rare diseases, this helps encourage companies to develop treatments by giving them a limited time to recover their costs without competition.
- Faster translation to patients: Since repurposed drugs have already passed safety testing for other uses, they can often move more quickly into clinical trials for new indications. This accelerates the timeline for potential treatments, providing hope for patients and families who have waited too long.
In the case of OCNDS, where the disease mechanisms are still being explored, drug repurposing allows researchers to build on existing knowledge from similar conditions that may share overlapping molecular or cellular features.
Real-World Examples
1. Rett Syndrome
At Vanderbilt University Medical Center, researchers are exploring the potential of three repurposed drugs – ketamine, vorinostat, and donepezil – for treating Rett syndrome, a rare neurodevelopmental disorder. These FDA approved drugs were originally treated for other conditions such as depression, cancer, and Alzheimer’s disease, but additional preliminary research suggests they may offer therapeutic benefits for Rett syndrome patients as well.
2. MAN1B1-CDG (congenital disorder of glycosylation (CDG)
Another example comes from MAN1B1-CDG. Research led by Dr. Clement Chow at the University of Utah has highlighted ibuprofen (Advil), a common over-the-counter pain reliever, as a potential therapy for MANB1-CDG through a drug-repurposing screen. With guidance from medical professionals, three MAN1B1-CDG families tried ibuprofen and carefully recorded changes. Within weeks, parents reported that their children’s keratosis pilaris (“chicken skin”) cleared up, social engagement increased, and communication improved - such as saying new words, using more speech, pointing to objects, and expressing themselves more. Some children also developed new skills, such as writing letters and brushing their teeth without help. When ibuprofen treatment was stopped, these improvements faded, suggesting the medication may have contributed to symptoms improvement. Stories like this provide hope that similar strategies could one day benefit families affected by OCNDS and other rare diseases.
Challenges to drug repurposing:
While drug repurposing offers many advantages, it also presents several challenges. Some key hurdles to this approach include:
- Choosing the correct disease target: Not every drug will work in every condition, even if the biology overlaps. A drug might act on a shared pathway, but how the pathway functions can vary depending on the disease. Scientists need a deep understanding of the disease’s underlying mechanisms to predict whether a drug will have a beneficial effect.
- Clinical trial design: Even if a drug is already approved for another use, scientists must still show that the drug is safe and effective in the new condition. This requires carefully designing new clinical trials to evaluate safety, dosing, and effectiveness in a different group of patients and requires funding to support the trial.
- Intellectual property and investment hurdles: Many repurposed drugs are off patent, meaning companies can’t exclusively sell them. This limits the potential profit and can make it difficult to justify the high cost of new clinical trials. Without a clear financial return, some companies may not invest in developing the drug for a new use. In these cases, public funding, nonprofit support, and rare disease advocacy are critical to advance the research forward.
Looking ahead:
Although drug repurposing comes with its own set of challenges, it remains a hopeful and practical approach in the search for rare disease treatments. By leveraging existing knowledge and previously approved therapies, this approach can accelerate progress, reduce development risks, and most importantly, bring new treatment options to patients faster.
For the OCNDS and rare disease community, drug repurposing represents a promising step forward in the journey toward effective and accessible therapies. Ongoing research efforts are currently exploring this approach for OCNDS. A study led by Dr. Vishnu Cuddapah (Baylor College of Medicine) is working to identify drugs that may help treat sleep and circadian rhythm disturbances associated with the disorder. In parallel, Dr. Clement Chow (University of Utah) is screening compounds to find candidates that could improve a broader range of OCNDS-related symptoms. Additionally, Unravel Biosciences is conducting a pilot study using RNA sequencing from individuals with OCNDS to identify potential therapies, focusing on the most common mutation, K198R.
Together, these studies reflect a growing commitment to advancing therapeutic options for the OCNDS community through the strategic use of drug repurposing.
References
Pushpakom, S., Iorio, F., Eyers, P. A., Escott, K. J., Hopper, S., Wells, A., Doig, A., Guilliams, T., Latimer, J., McNamee, C., Norris, A., Sanseau, P., Cavalla, D., & Pirmohamed, M. (2018). Drug repurposing: Progress, challenges and recommendations. In Nature Reviews Drug Discovery (Vol. 18, Issue 1). https://doi.org/10.1038/nrd.2018.168
Roessler, H. I., Knoers, N. V. A. M., van Haelst, M. M., & van Haaften, G. (2021). Drug Repurposing for Rare Diseases. In Trends in Pharmacological Sciences (Vol. 42, Issue 4). https://doi.org/10.1016/j.tips.2021.01.003
https://www.csnk2a1foundation.org/project-drug-repurposing-screen-using-ocnds-fly-model
https://perlara.substack.com/p/n-of-3-study-of-ibuprofen-for-man1b1?utm_source=publication-search
Figure made using BioRender
