Science Snapshot - Understanding Side Effects from Medications
Author: Ingrid Vallee, PharmD, PhD, CSNK2A1 Foundation Volunteer Writer
Editor: Gabrielle Rushing, PhD, CSNK2A1 Foundation Chief Scientific Officer
CSNK2A1 Foundation Parent Advisory Board Reviewers: Penelope Gatlin, Eric Finn, and Claire Whitehill
In this blog, we break down what “side effects” really mean, how they differ from adverse effects, and how to assess the risk-benefit balance of a treatment.
Introduction
When starting a new treatment—especially one that may affect the brain—it’s normal to have questions about safety and what kinds of effects to expect. Whether a treatment is appropriate is defined by two major criteria1:
- Safety: is the drug safe to take? These studies are meant to determine the risks associated with taking the drug and the acceptable level of side effects. What is considered “acceptable” is usually defined by regulatory agencies (such as the FDA) in consultation with medical experts and is weighed against the potential benefits of the drug for patients. However, what feels “acceptable” can also differ for each person or family, depending on their individual circumstances, values, and tolerance for risk.
- Efficacy: does the drug work and reduce expected symptoms? This evaluates the capacity of a drug to produce a particular benefit under ideal conditions.
These questions guide how medications are tested, approved, and monitored over time. When a drug is under consideration to be put on the market, both criteria are thoroughly studied in controlled groups of selected individuals, where the safety trials are conducted first. Safety and efficacy are then considered together under a common criterion called risk-benefit assessment: does the benefit of the drug outweigh the risk? Are the side effects acceptable considering that benefit?
After a drug is put on the market and becomes available to the public, this risk-benefit criterion is still updated regularly. Effectiveness is measured as the “real life” risk-benefit assessment, meaning how effective the drug is at targeting major symptoms versus causing side effects.
*Important Note: Health professionals and patients are encouraged to report side effects through the FDA’s online portal
MedWatch which also provides safety alerts.
Definitions:
A Side effect is defined as an unintended effect of a drug, occurring in addition to its desired or primary therapeutic effect. These effects can range in severity from minor annoyances to severe, life-threatening complications. Side effects are expected and can be beneficial or harmful2. They are often tolerable and can be used therapeutically. When a drug is approved to be put on the market, side effects may be known and they are tracked throughout the life cycle of the drug. For example, antihistamines such as Benadryl are intended to treat allergies, but one of their side effects is drowsiness. This drowsiness can also be harnessed therapeutically as a sleep aid. Side effects are expected to resolve on their own or to be tolerable, thus not requiring any intervention. When we consider side effects of certain drugs, two main effects can be described: adaptation effects and side effects. We discuss the details below.
Adverse events/effects are harmful, unintended, and undesired responses that occur during its appropriate use. They are not predictable, harmful (ranging from mild to severe), and can even be life threatening. Due to the potential severity and the unpredictability of the effects, they typically require immediate attention and management. An example of an adverse effect is an allergic reaction leading to anaphylaxis (severe allergic reaction that blocks the ability to breathe), which is life threatening. Different types of adverse effects can occur, depending on the cause, manifestation, and outcomes. More details can be found
here3.
Real World Example: side effects and psychotropic agents
Psychotropic agents are drugs or other substances that affect how the brain works and may cause changes in mood, awareness, thoughts, feelings, or behavior4. The main types include antidepressants, anti-anxiety medications, stimulants, antipsychotics, and mood stabilizers. A common example is diazepam (brand name Valium®).
Like many medicines, psychotropic drugs can cause side effects. Since they act directly on the brain, these drugs are more likely than others to produce noticeable side effects. Broadly, these effects can be grouped into two categories:
- Persistent side effects: These occur throughout the full course of treatment and are usually well known and documented. One example is diazepam, which is typically used to treat anxiety, alcohol withdrawal, muscle spasms, and seizures. As a benzodiazepine, it works by calming abnormal overactivity in the brain. Diazepam is known to cause drowsiness in some people and this persists as long as the drug is being used5.
- Initial or transient side effects (also called adaptation effects): Initial/transient side effects occur during the first few days to weeks starting the drug and often improve as the body adjusts to the drug. This happens when taking medicines that are intended to be taken over the course of a longer treatment, such as Fluoxetine (Prozac®). Prozac® is a selective serotonin reuptake inhibitor (SSRI) used to treat a wide range of mental health conditions. It is one of the most commonly prescribed antidepressants. Prozac® is known to cause nausea at the beginning of the treatment for some people, which usually goes away over time6.
It is important to remember that these categories are not always clear-cut. Some symptoms may overlap, and it can be hard to predict whether they will persist or improve. Every medication carries both benefits and risks, so discussing side effects with a healthcare professional before and during treatment can help families know what to expect.
What should families know?
Tracking symptoms before and after starting a new medication can help patients, families, and healthcare providers decide whether the benefits outweigh any side effects and if the treatment remains acceptable over time.
Here is an example flow chart of how the process of trying a new drug may work:
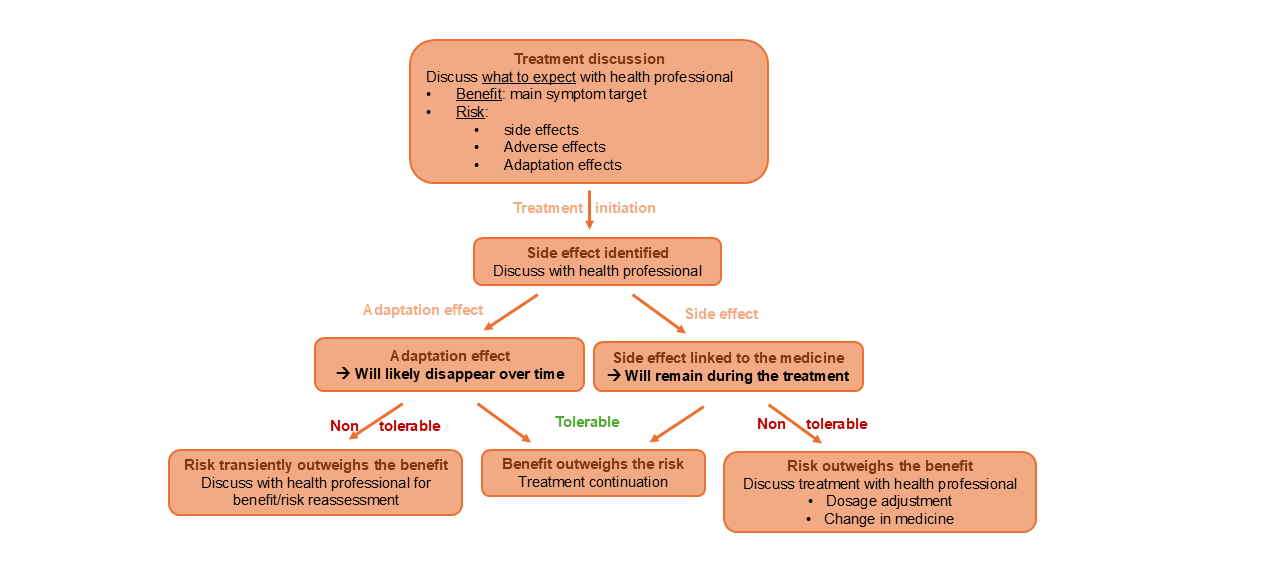
This table summarizes the different types of side effects discussed:
| Type of effect | Adverse Drug Reaction | Side Effect | Adaptation Effect |
|---|---|---|---|
| Definition | Unfavorable, unintended, severe | Beneficial or harmful, expected, mild | Beneficial or harmful, expected |
| Timeline | Rapidly triggered by drug use | Associated with drug use | Transient (improve after days to weeks of taking the drug) |
| Example | Severe allergic reaction to the drug | Antihistamine and drowsiness | Prozac® and nausea |
References:
- Evaluating Drug Efficacy and Safety - Clinical Pharmacology. Merck Manual Professional Edition https://www.merckmanuals.com/professional/clinical-pharmacology/concepts-in-pharmacotherapy/evaluating-drug-efficacy-and-safety.
- Sanchez, K. Adverse Reaction vs. Side Effect: What Are the Differences? GoodRx https://www.goodrx.com/drugs/side-effects/vs-adverse-reaction.
- Adverse Drug Reactions - Clinical Pharmacology. Merck Manual Professional Edition https://www.merckmanuals.com/professional/clinical-pharmacology/concepts-in-pharmacotherapy/evaluating-drug-efficacy-and-safety.
- Definition of psychotropic substance - NCI Dictionary of Cancer Terms - NCI. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/psychotropic-substance (2011).
- Diazepam: MedlinePlus Drug Information. https://medlineplus.gov/druginfo/meds/a682047.html.
- BCPS, S. P. B., PharmD. 12 Prozac (Fluoxetine) Side Effects: Insomnia, Nausea, and More. GoodRx https://www.goodrx.com/prozac/prozac-side-effects.
